Welcome to the website of the Vorselen Lab of mechanobiology and quantitative immune cell biology!
What we do
In the Vorselen Lab, we are fascinated by regulation of cell functions by physical forces. Immune cells encounter an enormous variety of threats, from small rigid bacteria to malleable cancer cells and irregularly shaped microplastics. Beyond biochemical signals, the diverse physical properties of such targets have emerged as key regulators of immune responses. In our lab we focus on innate immune cells, which are the first responders to threats and which use biophysical cues to guide target selection and enhance target killing. We use a profoundly interdisciplinary approach, combining development of controlled microparticles to interrogate immune functions, with quantitative imaging, molecular biology approaches and development of new biophysical approaches.
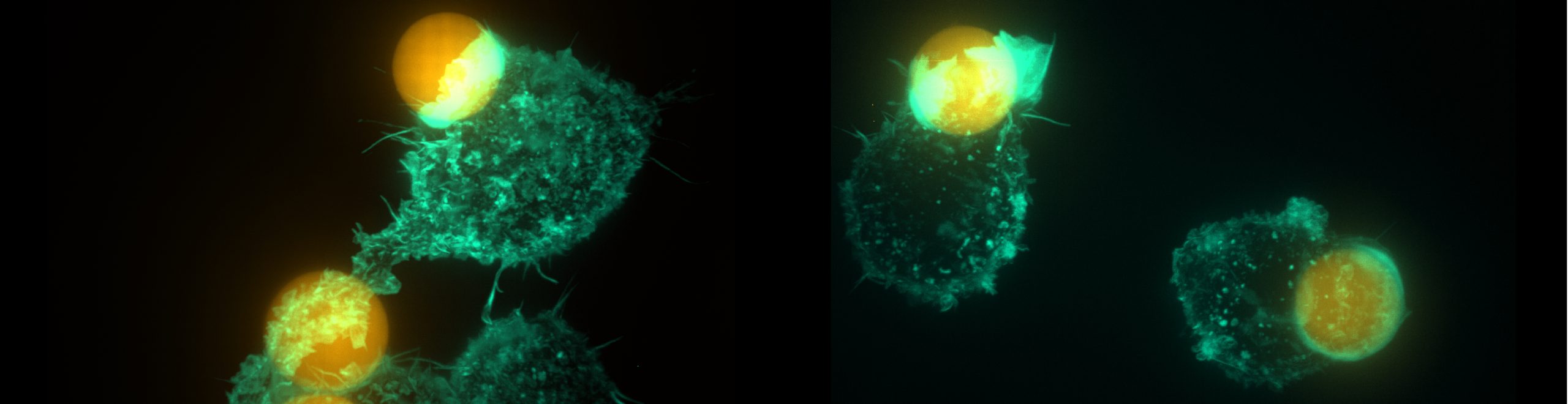
A special focus of our lab is phagocytosis, or eating by cells. It is a key function of innate immune cells and a thereapeutic target in some of the most pressing health issues, including neurodegeration, atherosclerosis and cancer. Understanding how macrophages respond to specific properties of these targets, will be critical to inform future targeted therapeutic strategies. From a biophysical perspective, phagocytosis is a fast, highly dynamic process that integrates active force generation by the cytoskeleton, membrane bending, cortex mechanics, adhesive forces and mechanosensing. The fundamental mechanobiology lessons we can learn from phagocytosis broadly translate to other innate immune processes, cell motility and other cell-cell interactions.
We develop new biophysical approaches to tune the physical input that cells receive and to measure cellular forces. A key contribution we made is the development of a “stress ball for the cell”: soft hydrogel microspheres that can be functionalized to trigger a variety of immune responses. They are tunable, uniquely model key physical characteristics of cancer cells (other models are up to 10 million-fold more rigid), and can be kneaded and squeezed by cells, rendering them as cellular force sensors. We combine these techniques with quantitative microscopy and cellular perturbations (genetic, pharmaceutical) to interrogate immune pathways. Together, this provides a detailed quantitative readout of immune cell behavior and helps us understand the regulation of immune processes by physical forces.
–> For more information see research. Interested in joining, see join!