Research
Development of new mechanobiology approaches
Although less familiar than genetic or biochemical aspects, physical forces have in the last two decades emerged as key regulators of cellular processes. We are interested in mechanical regulation of cell functions, specifically in our immune system, where immune cells use forces to guide target selection and enhance target killing. Understanding the role of forces in cellular functions (mechanobiology) critically depends on tools to measure cellular forces and modulate the mechanical signals received by cells. We developed deformable microspheres, which greatly expand the range of physiological contexts where such biophysical measurements can be made. This includes immune cell-target interactions, but also multicellular tissue and even live animals (in vivo). Multiple technological challenges lie ahead to realize this full potential: more detailed and complete mechanical measurements can be achieved and will be required to measure force exertion by single cells in the native tissue context.
Dynamics of immune effector functions:
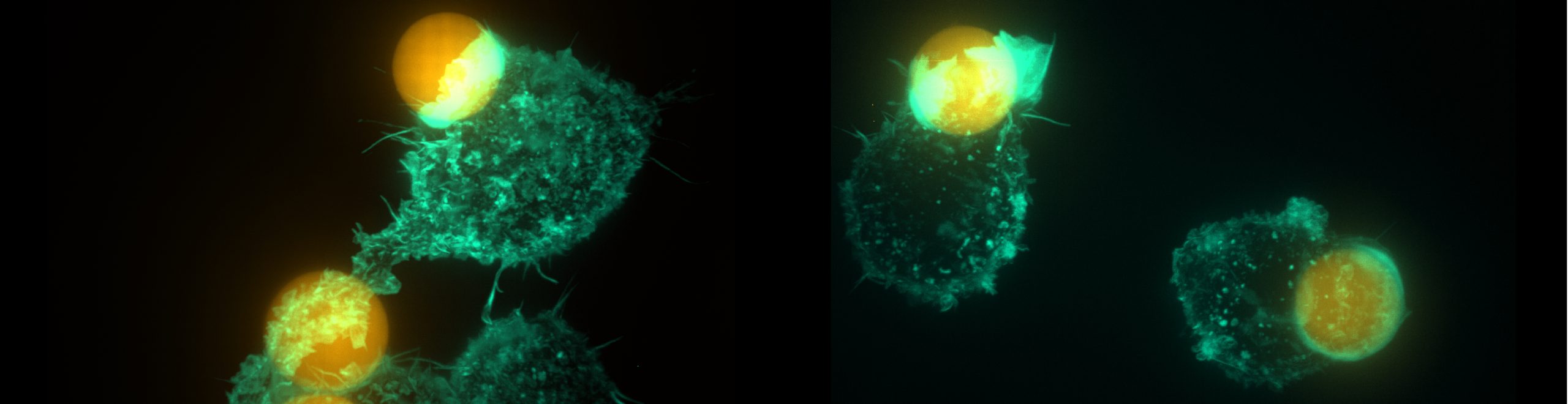
Interactions between immune cells and their targets often require large-scale changes of cellular shape and organization. These changes are rapid and adaptable to the target, yet very robust. We aim to understand how cells achieve this combination of adaptability and robustness. We use tuneable synthetic model targets for probing immune behavior, which allows us to determine how immune cells tune their responses based on traits of the prey (size, rigidity, identity and concentration of ligands). They also enable unusually quantitative measurements of immune cell processes, including measurement of force exertion patterns and distribution of proteins. By combining these measurements with cellular perturbations, we determine precisely how, where and when individual proteins act and contribute to organizing immune responses. So far, we have largely focused on phagocytosis by macrophages, where we revealed multiple new aspects, including a signature role for target constriction during target engulfment and the occurrence of protrusive teeth (see the movie). Currently, we are focusing on testing the role of an ignored force-component in phagocytosis, which has the potential to reveal a simple and robust mechanism of phagocytosis that is fundamentally distinct from current models. We are also expanding our investigations to other immune cell types!
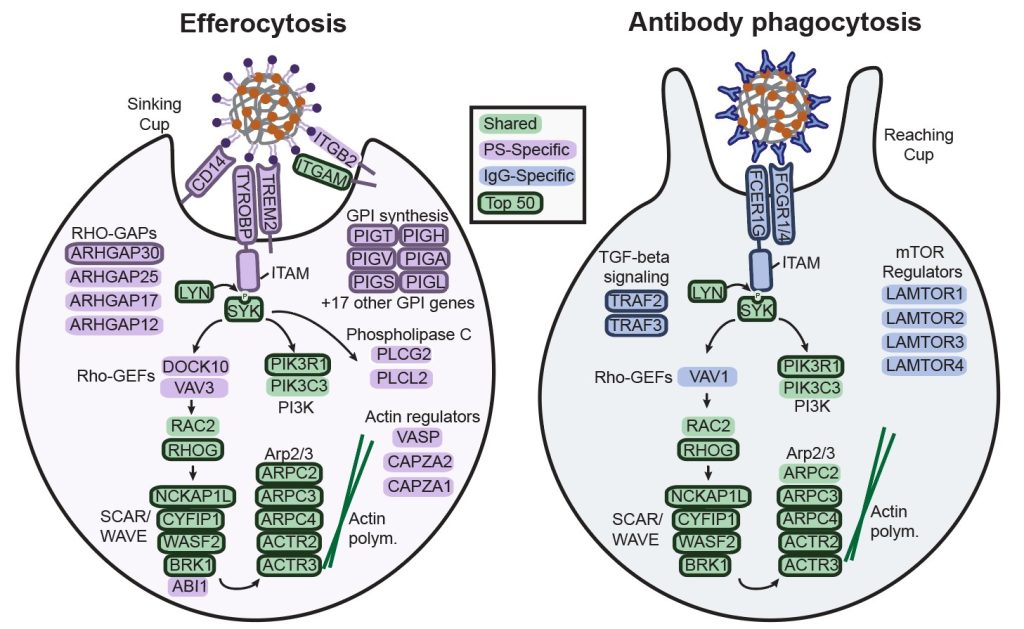
Molecular regulation of efferocytosis:
A current focus is on how macrophages clean up dead cells from tissues. They do this by engulfing and subsequently degrading them in a process called efferocytosis. This process is increasingly recognized as a target for therapeutic intervention in the context of atherosclerosis, neurodegenerative disease, and cancer. This form of engulfment is particularly challenging for immune cells, because dying cells are close in their surface chemistry and physical properties to healthy tissue cells. We recently showed that phosphatidylserine, the key trigger for sinking phagocytosis, induces unique phagocytic dynamics, including partial uptake of targets. We further performed a genome-wide CRISPR screen to identify regulators of this process. Now, we aim to reveal how known and novel regulators, including receptors, precisely function in efferocytosis.